Offer protection with the protein-based
Novavax COVID‑19 Vaccine, Adjuvanted
(2023-2024 Formula)1
Novavax COVID‑19 Vaccine, Adjuvanted
(2023-2024 Formula)1
The effectiveness of the Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) is based on the effectiveness of the Novavax COVID-19 vaccine (original monovalent) and the immunogenicity of the monovalent vaccine (Omicron BA.1) and the monovalent vaccine (Omicron BA.5).1
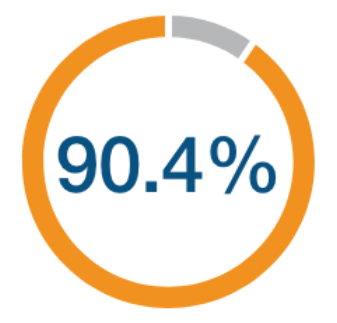
(95% CI: 83.8, 94.3)
N=25,657
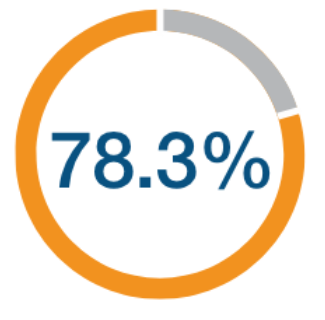
(95% CI: 37.6, 92.5)
N=1,799
Note: Data based on strains circulating at the time of the study.1
(95% CI: 87.0, 100.0)
N=25,452
high-risk patients2*†
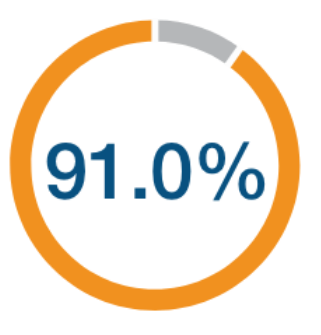
(95% CI: 83.6, 95.0)
N=24,230
The Novavax COVID‑19 vaccine may not protect all vaccine recipients1
Post hoc analysis
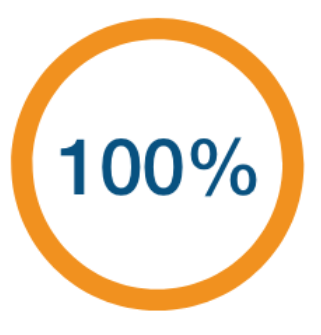
In a phase 3 trial analyzing COVID‑19 hospitalizations, 4 hospitalizations were identified—0 among Novavax COVID‑19 vaccine recipients and 4 among placebo—resulting in a vaccine efficacy against hospitalization of 100% (95% Cl: 28.8, 100.0).3
- Limitations include vaccine efficacy estimates impacted by a low number of events (ie, hospitalizations), pandemic-era restrictions successfully reducing infections and hospitalizations during the study, and the exclusion of key demographics, such as participants with certain underlying health conditions3
(95% Cl: 28.8, 100.0)
N=25,482
Study design1
The phase 3 clinical study is a multicenter, randomized, observer-blinded, placebo-controlled study conducted in the United States and Mexico. Upon enrollment, participants were stratified by age (18 to 64 years; ≥65 years) and randomized in a 2:1 ratio to receive the Novavax COVID‑19 vaccine or placebo. Participants 12 to 17 years of age were included in the expansion trial.
Inclusion criteria: Participants with clinically stable underlying comorbidities were included, as well as those with well-controlled HIV.
Exclusion criteria: Participants were excluded if they were significantly immunocompromised due to immunodeficiency disease or on chemotherapy for active cancer, had received chronic immunosuppressive therapy or received immunoglobulin or blood-derived products within 90 days, were pregnant or breastfeeding, and/or had a history of laboratory-confirmed, diagnosed COVID‑19.
In this follow-up evaluation before the booster dose, it was predetermined that participants who received the primary vaccination series pre-crossover would receive a Novavax COVID‑19 Vaccine, Adjuvanted booster at the 11-month mark. Those who received placebo pre-crossover were not included in this analysis. The 11-month antibody levels were based on evaluation of anti-prototype spike antibodies to the Novavax COVID‑19 vaccine.4,5
A booster response with the Novavax COVID‑19 vaccine was observed in a phase 3 clinical trial with neutralizing antibodies 3.4x higher than after the primary series1
Geometric mean titer (GMT) ratio of neutralizing antibody titers (MN50) against the original SARS-CoV-2 strain at 28 days after a booster dose vs 14 days after primary series
- The analysis of the GMT ratio of MN50 following the booster dose vs the primary series met the noninferiority criteria for a booster response (lower limit of the 95% CI >0.67 and point estimate >0.83)
- The lower limit of the two-sided 95% CI for the difference in seroconversion rate (SCR) of the booster dose vs primary series was -14.4%, which did not meet the noninferiority criteria for a booster response (lower limit of 95% CI for the percentage difference of ≥-10%)
- An additional post hoc analysis evaluated SCR using baseline MN50 prior to dose 1 of the primary series. The booster dose SCR, with seroconversion defined as at least a 4-fold rise relative to the time of the first dose, was 98.3%. The difference in SCR among 239 participants in this post hoc analysis who had immunogenicity data (microneutralization) available for both the booster and primary series was 3.8% (95% CI: 2.0, 7.0)
Note: The median duration between the time of the second dose of the Novavax COVID‑19 vaccine and the time of the booster dose was 10 months.1
A booster response with the Novavax COVID‑19 vaccine was observed in a phase 3 clinical trial with neutralizing antibodies 2.7x higher than after the primary series1
Geometric mean titer (GMT) ratio of neutralizing antibody titers (MN50) against the original SARS-CoV-2 strain at 28 days after a booster dose vs 14 days after primary series
- The analysis of the GMT ratio of MN50 following the booster dose vs the primary series met the noninferiority criteria for a booster response (lower limit of the 95% CI >0.67 and point estimate >0.83)
- The lower limit of the two-sided 95% CI for the difference in seroconversion rate (SCR) of the booster dose vs primary series was -6.8%, which met the noninferiority criteria for a booster response (lower limit of 95% CI for the percentage difference of ≥-10%)
Note: The median duration between the time of the second dose of the Novavax COVID‑19 vaccine and the time of the booster dose was 10.6 months.1
Study design1
The open-label booster phase of this study included participants 18 years and older in the United States and Mexico and participants 12 to 17 years of age in the United States, who received a single booster dose of the Novavax COVID-19 vaccine. Participants aged 18 years of age and older received a booster at least 6 months after completion of the primary series and participants 12 to 17 years of age received a booster at least 5 months after completion of the primary series.
Prespecified immunogenicity noninferiority analyses included an assessment of GMT ratio of MN50 and difference in SCR. Seroconversion for a participant was defined as achieving a 4-fold rise in MN50 from baseline (before the booster dose and before the first dose of the primary series).
References: 1. Novavax COVID‑19 Vaccine, Adjuvanted (2023-2024 Formula) EUA Fact Sheet for Healthcare Providers. Novavax, Inc.; October 2023. 2. Dunkle LM, Kotloff CL, et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med. 2022;386(6):531-543. 3. Marchese AM, Zhou X, et al. NVX-CoV2373 vaccine efficacy against hospitalization: a post hoc analysis of the PREVENT-19 phase 3, randomized, placebo-controlled trial. Vaccine. 2023;41(22):3461-3466. 4. Añez G, Bennett C. Paving the way for protein: Novavax’s COVID‑19 vaccine as a booster and variant-adapted. Presented at: World Vaccine Congress Europe; October 12, 2022; Barcelona, Spain. 5. Data on file. Novavax, Inc. 2023.




